Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohira, Saki; Abe, Takeyasu; Iida, Yoshihisa
Radiochimica Acta, 111(7), p.525 - 531, 2023/07
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)The solubility of 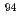 Nb in calcium alkaline solutions is one of the important parameters in safety assessment of intermediate-depth disposal which are assumed to use cementitious materials. Nb solubility and solubility-limiting solid phases of Nb in these systems remain unclear. The oversaturation solubility experiments were performed systematically in the 0.001-0.1 M CaCl
Nb in calcium alkaline solutions is one of the important parameters in safety assessment of intermediate-depth disposal which are assumed to use cementitious materials. Nb solubility and solubility-limiting solid phases of Nb in these systems remain unclear. The oversaturation solubility experiments were performed systematically in the 0.001-0.1 M CaCl solutions under alkali conditions, and the characterization of precipitated solid phase controlling Nb solubility was conducted. The negative dependence of Nb solubilities on pH and Ca concentration was observed in solubility experiments, the molar ratio of Nb to Ca of precipitated solid phase was 0.66. The pH and Ca dependence of Nb solubilities was reproduced by the reaction with Nb aqueous species Nb(OH)
solutions under alkali conditions, and the characterization of precipitated solid phase controlling Nb solubility was conducted. The negative dependence of Nb solubilities on pH and Ca concentration was observed in solubility experiments, the molar ratio of Nb to Ca of precipitated solid phase was 0.66. The pH and Ca dependence of Nb solubilities was reproduced by the reaction with Nb aqueous species Nb(OH)
 and Ca-Nb oxide with the molar ratio of Nb to Ca 0.66, e.g., Ca
and Ca-Nb oxide with the molar ratio of Nb to Ca 0.66, e.g., Ca Nb
Nb O
O (am).
(am).
Sato, Tetsuya; Nagame, Yuichiro*
Radiochimica Acta, 110(6-9), p.441 - 451, 2022/07
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)We describe our recent achievements in the effective production of low-energy ion-beams of the elements at the end of the actinide series, fermium (Fm, atomic number Z = 100), mendelevium (Md, Z = 101), nobelium (No, Z = 102), and lawrencium (Lr, Z = 103), using a surface ionization ion-source installed in the ISOL (Isotope Separator On-Line) at the Tandem accelerator facility of JAEA (Japan Atomic Energy Agency). Then the successful measurements of the first ionization potentials (IP ) of these elements with the ISOL setup are reviewed. The measured IP
) of these elements with the ISOL setup are reviewed. The measured IP values increased up to No via Fm and Md, while that of Lr was the lowest among the actinides. Based on the variation of the IP1 values of the heavy actinides with the atomic number in comparison with those of the heavy lanthanides, the results clearly demonstrated that the 5f orbitals are fully filled at No, and the actinide series ends with Lr. Furthermore, the IP
values increased up to No via Fm and Md, while that of Lr was the lowest among the actinides. Based on the variation of the IP1 values of the heavy actinides with the atomic number in comparison with those of the heavy lanthanides, the results clearly demonstrated that the 5f orbitals are fully filled at No, and the actinide series ends with Lr. Furthermore, the IP value of Lr provoked controversy over its position in the Periodic Table, so a short introduction to this issue is presented. The feasibility of the extension of chemical studies to still heavier elements with their ion-beams generated by ISOL is briefly discussed.
value of Lr provoked controversy over its position in the Periodic Table, so a short introduction to this issue is presented. The feasibility of the extension of chemical studies to still heavier elements with their ion-beams generated by ISOL is briefly discussed.
G tz, M.*; Yakushev, A.*; G
tz, M.*; Yakushev, A.*; G tz, S.*; Di Nitto, A.*; D
tz, S.*; Di Nitto, A.*; D llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
Radiochimica Acta, 110(2), p.75 - 86, 2022/02
Times Cited Count:2 Percentile:31.78(Chemistry, Inorganic & Nuclear)The study of volatile superheavy element carbonyl complexes requires more efficient methods because the yield of transactinide elements decreases with increasing atomic number. This is achieved by using a newly developed double chamber system to separate the recoil chamber and the reaction one, thereby avoiding the decomposition of reactive molecules by the projectile ion beam, which hinders the synthesis of carbonyl complexes. The feasibility of this method was verified by synthesizing 5d metal short-lived isotopes as homologous element isotopes of the light transactinide elements Sg, Bh, Hs, and Mt at the Japan Atomic Energy Agency tandem accelerator and conducting model experiments.
Hemmi, Ko; Walker, A.*; Yamaguchi, Tetsuji
Radiochimica Acta, 109(7), p.539 - 546, 2021/07
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)Plutonium(IV) sorption onto quartz in carbonate solutions was systematically investigated under anaerobic conditions to analyze the sorption behaviors of Pu(IV) with a non-electrostatic model (NEM). Pu(IV) sorption data was obtained from batch sorption experiments as a function of pH and carbonate concentration. The Pu(IV) sorption onto quartz showed similar tendencies to Th(IV), which is considered to be chemically analogous as a tetravalent actinoid. The distribution coefficient,  d, of Pu(IV) onto quartz showed inverse proportionality to the square of the total carbonate concentration under the investigated pH conditions of 8 to 11. The modeling study, however, revealed a Th(IV) sorption model, which is
d, of Pu(IV) onto quartz showed inverse proportionality to the square of the total carbonate concentration under the investigated pH conditions of 8 to 11. The modeling study, however, revealed a Th(IV) sorption model, which is  SOTh(OH)
SOTh(OH)
 and
and  SOThOH(CO
SOThOH(CO )
)
 , could not be applied to simulate the Pu(IV) sorption onto quartz. It was inferred that the electrostatic repulsion between negatively charged ligands limited the formation of
, could not be applied to simulate the Pu(IV) sorption onto quartz. It was inferred that the electrostatic repulsion between negatively charged ligands limited the formation of  SOM(OH)
SOM(OH)
 and
and  SOMOH(CO
SOMOH(CO )
)
 for Pu(IV) with smaller ionic radii than Th(IV). The Pu(IV) sorption model was developed as
for Pu(IV) with smaller ionic radii than Th(IV). The Pu(IV) sorption model was developed as  SOPu(OH)
SOPu(OH) and
and  SOPu(OH)
SOPu(OH)
 . In addition, data of Pu(IV) sorption onto muscovite was obtained in order to be compared with data for quartz.
. In addition, data of Pu(IV) sorption onto muscovite was obtained in order to be compared with data for quartz.
G tz, M.*; G
tz, M.*; G tz, S.*; Kratz, J.-V.*; Ballof, J.*; D
tz, S.*; Kratz, J.-V.*; Ballof, J.*; D llmann, Ch. E.*; Eberhardt, K.*; Mokry, C.*; Renisch, D.*; Runke, J.*; Sato, Tetsuya; et al.
llmann, Ch. E.*; Eberhardt, K.*; Mokry, C.*; Renisch, D.*; Runke, J.*; Sato, Tetsuya; et al.
Radiochimica Acta, 109(3), p.153 - 165, 2021/03
Times Cited Count:3 Percentile:45.99(Chemistry, Inorganic & Nuclear)We report on a novel two-chamber approach for the synthesis of volatile complexes that allows spatial decoupling of thermalization and gas-phase carbonyl complex synthesis. Neutron induced fission on  U and spontaneous fission of
U and spontaneous fission of  Cm were employed for the production of the fission products. These were stopped inside a gas volume behind the target and flushed with an inert-gas flow into a second chamber. This was flushed with carbon monoxide to allow the gas-phase synthesis of carbonyl complexes. Parameter studies of the transfer from the first into the second chamber as well as on the carbonyl complex formation and transport processes have been performed. High overall efficiencies of more than 50% were reached rendering this approach interesting for studies of superheavy elements. Our results show that carbonyl complex formation of thermalized fission products is a single-atom reaction, and not a hot-atom reaction.
Cm were employed for the production of the fission products. These were stopped inside a gas volume behind the target and flushed with an inert-gas flow into a second chamber. This was flushed with carbon monoxide to allow the gas-phase synthesis of carbonyl complexes. Parameter studies of the transfer from the first into the second chamber as well as on the carbonyl complex formation and transport processes have been performed. High overall efficiencies of more than 50% were reached rendering this approach interesting for studies of superheavy elements. Our results show that carbonyl complex formation of thermalized fission products is a single-atom reaction, and not a hot-atom reaction.
Yamaguchi, Tetsuji; Ohira, Saki; Hemmi, Ko; Barr, L.; Shimada, Asako; Maeda, Toshikatsu; Iida, Yoshihisa
Radiochimica Acta, 108(11), p.873 - 877, 2020/11
Times Cited Count:7 Percentile:66.68(Chemistry, Inorganic & Nuclear) system
systemSasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Radiochimica Acta, 108(9), p.689 - 699, 2020/09
Times Cited Count:9 Percentile:75.92(Chemistry, Inorganic & Nuclear)The simultaneous separation of Am and Cm from lanthanides is important for atomic energy fields. All lanthanides, Am, and Cm can be extracted by diglycolamide (DGA). In addition, relatively high separation factors between An and Ln were obtained by the extraction system of TODGA, DTPA (diethylenetriamine-pentaacetic acid) and HNO . In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
. In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
Nakahara, Masaumi; Sano, Yuichi; Nomura, Kazunori
Radiochimica Acta, 108(9), p.701 - 706, 2020/09
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)To evaluate the corrosion behavior of a Pu evaporator made from Zr in a reprocessing plant, the influence of PuO
 was investigated with Pu nitrate solutions in electrochemical experiments. The maximum open circuit potential of Zr in the Pu nitrate solution was approximately 1 V in the Pu nitrate solution containing 7 mol dm
was investigated with Pu nitrate solutions in electrochemical experiments. The maximum open circuit potential of Zr in the Pu nitrate solution was approximately 1 V in the Pu nitrate solution containing 7 mol dm HNO
HNO . However, there were no significant changes at high PuO
. However, there were no significant changes at high PuO
 concentrations, and Zr showed high corrosion resistance under our experimental conditions.
concentrations, and Zr showed high corrosion resistance under our experimental conditions.
Koma, Yoshikazu; Murakami, Erina
Radiochimica Acta, 107(9-11), p.965 - 977, 2019/09
Times Cited Count:1 Percentile:11.15(Chemistry, Inorganic & Nuclear)Fukushima Daiichi Nuclear Power Station, which is owned by Tokyo Electric Power Company, suffered from the great earthquake and Tsunami on 11 March 2011, and serious contamination with radioactive nuclides occurred. To investigate methodologies of waste management, contaminated materials have been radiochemically analyzed. This paper reviews the analytical data for actinide elements. Actinide nuclides are detected in the contaminated water. The contaminated water is chemically decontaminated, although actinide concentration does not decrease with time. This suggests that actinides come from the damaged fuel with slow dissolution. From the topsoil at the site, Pu, Am and Cm were detected and come from the damaged fuel, whereas U from natural. TRU would slowly move to deeper. Contamination of rubble is nonuniform and actinides are detected as well as fission products. For vegetation, TRU nuclides were found from fallen leaves near the reactor buildings.
Nagame, Yuichiro
Radiochimica Acta, 107(9-11), p.803 - 819, 2019/09
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)Sch del, M.*; Nagame, Yuichiro
del, M.*; Nagame, Yuichiro
Radiochimica Acta, 107(7), p.561 - 585, 2019/07
Times Cited Count:3 Percentile:31.89(Chemistry, Inorganic & Nuclear) -HF mixture; Kinetics and mechanism
-HF mixture; Kinetics and mechanismSimonnet, M.; Barr , N.*; Drot, R.*; Le Naour, C.*; Sladkov, V.*; Delpech, S.*
, N.*; Drot, R.*; Le Naour, C.*; Sladkov, V.*; Delpech, S.*
Radiochimica Acta, 107(4), p.289 - 297, 2019/04
Times Cited Count:2 Percentile:21.58(Chemistry, Inorganic & Nuclear) acidic solutions
acidic solutionsYokoyama, Akihiko*; Kitayama, Yuta*; Fukuda, Yoshiki*; Kikunaga, Hidetoshi*; Murakami, Masashi*; Komori, Yukiko*; Yano, Shinya*; Haba, Hiromitsu*; Tsukada, Kazuaki; Toyoshima, Atsushi*
Radiochimica Acta, 107(1), p.27 - 32, 2019/01
Times Cited Count:1 Percentile:11.15(Chemistry, Inorganic & Nuclear) and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCA
and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCALens, L.*; Yakushev, A.*; D llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
Radiochimica Acta, 106(12), p.949 - 962, 2018/12
Times Cited Count:8 Percentile:62.99(Chemistry, Inorganic & Nuclear)Online gas-solid adsorption studies with single atom quantities of Hg, Tl, and Pb on SiO and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO
and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO
or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO surface at room temperature. On the other hand, the Hg did not adsorb on the SiO
surface at room temperature. On the other hand, the Hg did not adsorb on the SiO surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
 (am) in the aqueous K
(am) in the aqueous K - HCO
- HCO
 - CO
- CO
 - OH
- OH - H
- H O system
O systemRai, D.*; Kitamura, Akira; Rosso, K.*
Radiochimica Acta, 105(8), p.637 - 647, 2017/08
Times Cited Count:2 Percentile:19.65(Chemistry, Inorganic & Nuclear)Solubility of HfO (am) was determined as a function of KHCO
(am) was determined as a function of KHCO concentrations ranging from 0.001 mol.kg
concentrations ranging from 0.001 mol.kg to 0.1 mol.kg
to 0.1 mol.kg . The solubility of HfO
. The solubility of HfO (am) increased dramatically with the increase in KHCO
(am) increased dramatically with the increase in KHCO concentrations, indicating that Hf(IV) makes strong complexes with carbonate. Thermodynamic equilibrium constants for the formation of Hf-carbonate complexes were determined using both the Pitzer and SIT models. The dramatic increase in Hf concentrations with the increase in KHCO
concentrations, indicating that Hf(IV) makes strong complexes with carbonate. Thermodynamic equilibrium constants for the formation of Hf-carbonate complexes were determined using both the Pitzer and SIT models. The dramatic increase in Hf concentrations with the increase in KHCO concentrations can best be described by the formation of Hf(OH-)
concentrations can best be described by the formation of Hf(OH-) (CO
(CO )
)
 and Hf(CO
and Hf(CO )
)
 . The log
. The log K
K values for the reactions [Hf
values for the reactions [Hf + 2 CO
+ 2 CO
 +2 OH
+2 OH
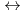 Hf(OH)
Hf(OH) (CO
(CO )
)
 ] and [Hf
] and [Hf + 5 CO
+ 5 CO

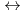 Hf(CO
Hf(CO )
)
 ], based on the SIT model, were determined to be 44.53
], based on the SIT model, were determined to be 44.53  0.46 and 41.53
0.46 and 41.53  0.46, respectively.
0.46, respectively.
 (cr)
(cr)Rai, D.*; Kitamura, Akira; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Radiochimica Acta, 104(8), p.583 - 592, 2016/08
Times Cited Count:5 Percentile:43.41(Chemistry, Inorganic & Nuclear)Solubility studies were conducted with HfO (cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400
(cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400  C, and (4) heating amorphous HfO
C, and (4) heating amorphous HfO (am) suspensions to 90
(am) suspensions to 90  C to ascertain whether the HfO
C to ascertain whether the HfO (am) converts to HfO
(am) converts to HfO (cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO
(cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO (cr) contains a small fraction of less crystalline, but not amorphous, material [HfO
(cr) contains a small fraction of less crystalline, but not amorphous, material [HfO (lcr)] and this, rather than the HfO
(lcr)] and this, rather than the HfO (cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log
(cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log of the solubility product of HfO
of the solubility product of HfO (cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
(cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
 and W(CO)
and W(CO)
Usoltsev, I.*; Eichler, R.*; Wang, Y.*; Even, J.*; Yakushev, A.*; Haba, Hiromitsu*; Asai, Masato; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; et al.
llmann, Ch. E.*; et al.
Radiochimica Acta, 104(3), p.141 - 151, 2016/03
Times Cited Count:31 Percentile:95.03(Chemistry, Inorganic & Nuclear)Conditions of the production and decomposition of hexacarbonyl complexes of short-lived Mo and W isotopes were investigated to study thermal stability of the heaviest group 6 hexacarbonyl complex Sg(CO) . A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO)
. A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO) and W(CO)
and W(CO) , indicating that the first bond dissociation energy of Sg(CO)
, indicating that the first bond dissociation energy of Sg(CO) could be determined with this technique.
could be determined with this technique.
 , and NaCO
, and NaCO solutions
solutionsUehara, Akihiro*; Fujii, Toshiyuki*; Yamana, Hajimu*; Okamoto, Yoshihiro
Radiochimica Acta, 104(1), p.1 - 9, 2016/01
Times Cited Count:7 Percentile:55.03(Chemistry, Inorganic & Nuclear)The spectroelectrochemical cell was fabricated for in-situ X-ray absorption fine structure (XAFS) measurement. The XAFS spectra of U L -edge were monitored in electrolyte solutions during the electrochemical reduction. Tetravalent uranium was electrochemically prepared from hexavalent uranium and the extended X-ray absorption fine structure (EXAFS) was analyzed. The U
-edge were monitored in electrolyte solutions during the electrochemical reduction. Tetravalent uranium was electrochemically prepared from hexavalent uranium and the extended X-ray absorption fine structure (EXAFS) was analyzed. The U formed in 0.1M HNO
formed in 0.1M HNO was partially re-oxidized to UO
was partially re-oxidized to UO
 by NO
by NO
 in the solution. The UO
in the solution. The UO
 carbonates were prepared during electrolysis.
carbonates were prepared during electrolysis.
Yamaguchi, Tetsuji; Takeda, Seiji; Nishimura, Yuki; Iida, Yoshihisa; Tanaka, Tadao
Radiochimica Acta, 102(11), p.999 - 1008, 2014/11
Times Cited Count:1 Percentile:8.88(Chemistry, Inorganic & Nuclear)An attempt was made to select thermodynamic data with uncertainties and to evaluate the solubility of radioelements with uncertainties considering variation in groundwater chemistry. The thermodynamic data was selected by reviewing the JAEA-TDB released in 2012. Data for Nb, Pd and Pa were revised from the viewpoint of the consistency of the data selection process. Data for Se, U and Pa were revised from the viewpoint of the conservativeness. Up-to-date ternary calcium-metal(IV)-OH complexes were adopted for Zr, Th, U, Np and Pu. A Monte Carlo-based probabilistic calculation code, PA-SOL, was used for probabilistic analysis of the solubility.
 nitric acid at elevated temperatures
nitric acid at elevated temperaturesBan, Yasutoshi; Hakamatsuka, Yasuyuki; Tsutsui, Nao; Urabe, Shunichi; Hagiya, Hiromichi; Matsumura, Tatsuro
Radiochimica Acta, 102(9), p.775 - 780, 2014/09
Times Cited Count:5 Percentile:36.96(Chemistry, Inorganic & Nuclear)Optical absorption spectra of Np in 3 mol/dm nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI),
nitric acid at elevated temperatures were measured using an optical glass cell with a water jacket having a light path of 1 cm, and molar extinction coefficients of Np(VI), 
 , were obtained at various temperature. The values of
, were obtained at various temperature. The values of 
 was found to decrease with increasing the temperature, and could be described by the equation
was found to decrease with increasing the temperature, and could be described by the equation 
 = -0.14
= -0.14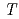 +85.5, where
+85.5, where 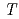 is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm
is the temperature. Oxidation of Np(V) to Np(VI) in 3 mol/dm nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)]
nitric acid at elevated temperatures was observed using the optical glass cell. Oxidation of Np(V) proceeded as pseudo-first order with respect to Np(V) concentration. The rate equation in the temperature range of 336-362 K was obtained as follows: -d[Np(V)] /dt = 2.2
/dt = 2.2 10
10 exp[-65
exp[-65 10
10 /(
/(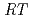 )][Np(V)]
)][Np(V)] , where
, where  and [Np(V)]
and [Np(V)] indicate the gas constant and Np(V) concentration at time
indicate the gas constant and Np(V) concentration at time 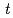 , respectively.
, respectively.